

In fact, a majority of the Usage Panel now accepts this usage. After all a precipitous increase in reports of measles is also an abrupt or sudden event. Many people object to this usage out of a desire to keep precipitate and precipitous distinct, but the extension of meaning from "steep" to "abrupt" is perfectly natural. But precipitous and precipitously are also frequently used to mean "abrupt, hasty," which takes them into territory that would ordinarily belong to precipitate and precipitately: their precipitous decision to leave. Precipitous currently means "steep" in both literal and figurative senses: the precipitous rapids of the upper river a precipitous drop in commodity prices. To precipitate is to form an insoluble compound, either by decreasing the solubility of a compound or by reacting two salt solutions.
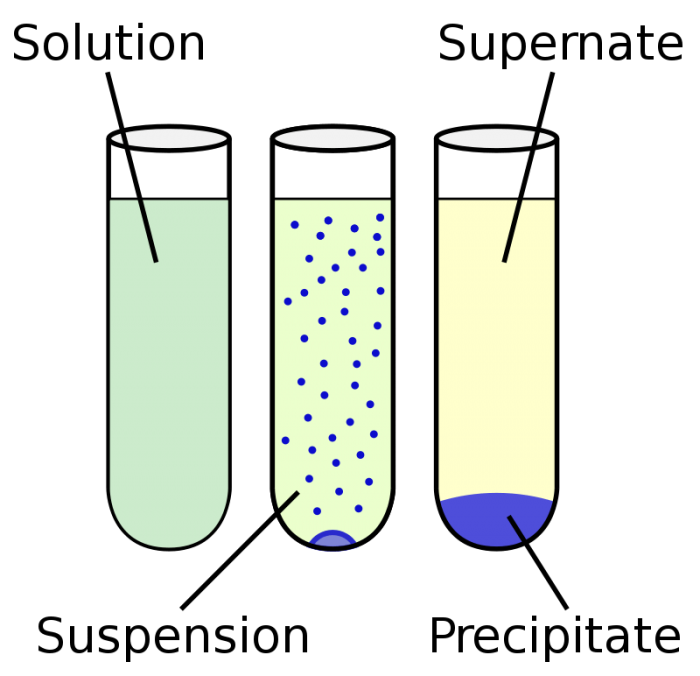
Substances with relatively low solubilities are. (Ionic salts are a good example: usually they have strong interactions in the solid and solvated states.Usage Note: The adjective precipitate and the adverb precipitately were once applied to physical steepness but are now used primarily of rash, headlong actions: Their precipitate entry into the foreign markets led to disaster. In chemistry, precipitate is both a verb and a noun. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. 1 Analogously, in medicine, coprecipitation is specifically the precipitation of an unbound 'antigen along with an antigen-antibody complex'.
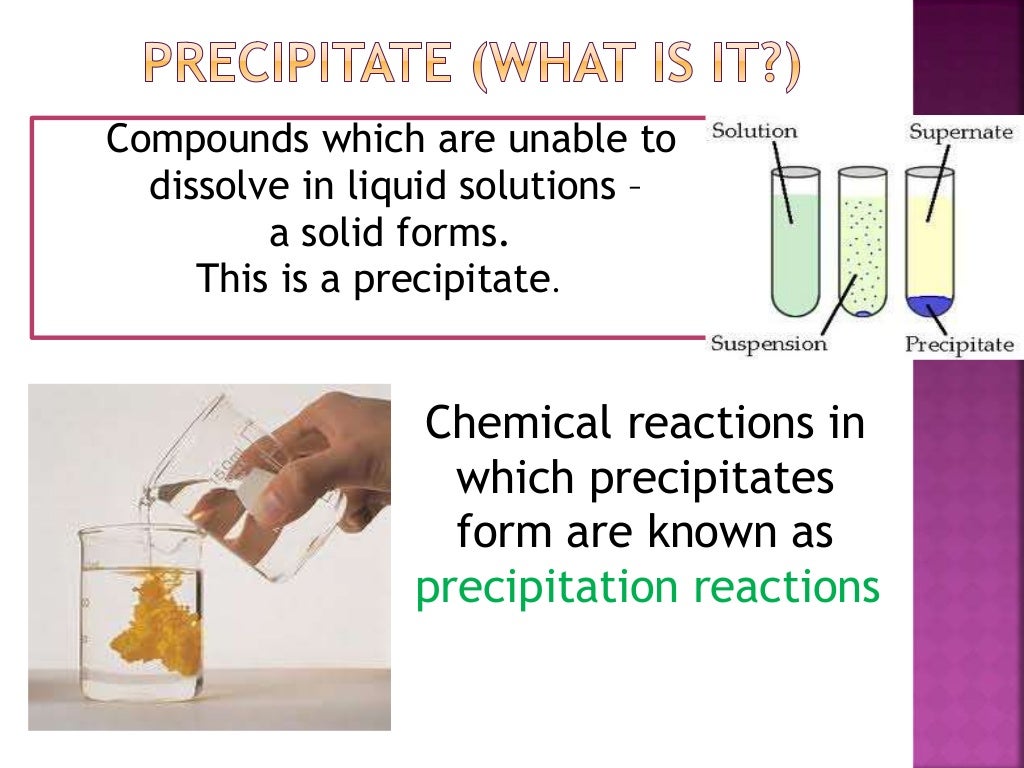
For instance, if it has very strong interactions between molecules or ions in the solid state, then it won't be very soluble unless the solvation interations are also very strong. In chemistry, coprecipitation ( CPT) or co-precipitation is the carrying down by a precipitate of substances normally soluble under the conditions employed. Solubility depends on the relative stability of the solid and solvated states for a particular compound. Chemical reactions which are characterised by the formation of insoluble solid substances are called precipitates. (It's also a little funny because many salts aren't strong electrolytes, so teachers might be telling their students to write an equation that doesn't show what's really happening.) However, it does help show what it means to be a spectator ion, since they are the same on both sides when you write it like this. Table 2 provides a summary of properties and considerations appropriate to chemicals commonly used for precipitation.
C Large amounts of chemicals may need to be transported to the treatment location. precipitate stresses lack of due deliberation and implies. No real chemist would be likely to do this because it is a nuisance. C The addition of treatment chemicals, especially lime, may increase the volume of waste sludge up to 50 percent. precipitate, headlong, abrupt, impetuous, sudden mean showing undue haste or unexpectedness.


 0 kommentar(er)
0 kommentar(er)
